Have you pondered the floating behavior of ice in water? For most people ice cubes and glaciers being on the surface of the water rather than underneath is usually just an observation. The question why ice floats in water, however, seems simple at first. In this article, I bring to you some astounding observations that can be simplified easily for understanding while debunking this phenomenon at the same time.
Key Takeaways
- The buoyancy principle explains that ice is in fact suspended on water due to the fact that ice has a lower liquid density in comparison to water.
- This is further caused by ice molecules forming spaces during freezing which expands them and minimizes weight in comparison to space taken.
- As the temperature decreases, water’s density goes down, thus allowing ice to rest on top without sinking.
- Because of its porous structure, ice floats and does not drown underneath.
- This porous structure is also the reason why buoyancy lets ice chill on the surface of water.
What Makes Ice Float in Water?
How come ice cubes can be seen easily in drinks while other solids drown when submerged? The reason behind this question is interlinked with not only the physics of an object but also density and buoyancy.
Step by step we will analyze the why and how behind the buoyant and freestanding behavior of ice.
Density: The Key to Understanding Why Ice Floats
The first portion that we need to tackle is density. Density defines what mass is packed in a particular volume. It essentially gives the measure of how heavy something is with respect to its volume. An object is heavier for its volume when it is denser, whereas an object is lighter for its size when it is less dense.
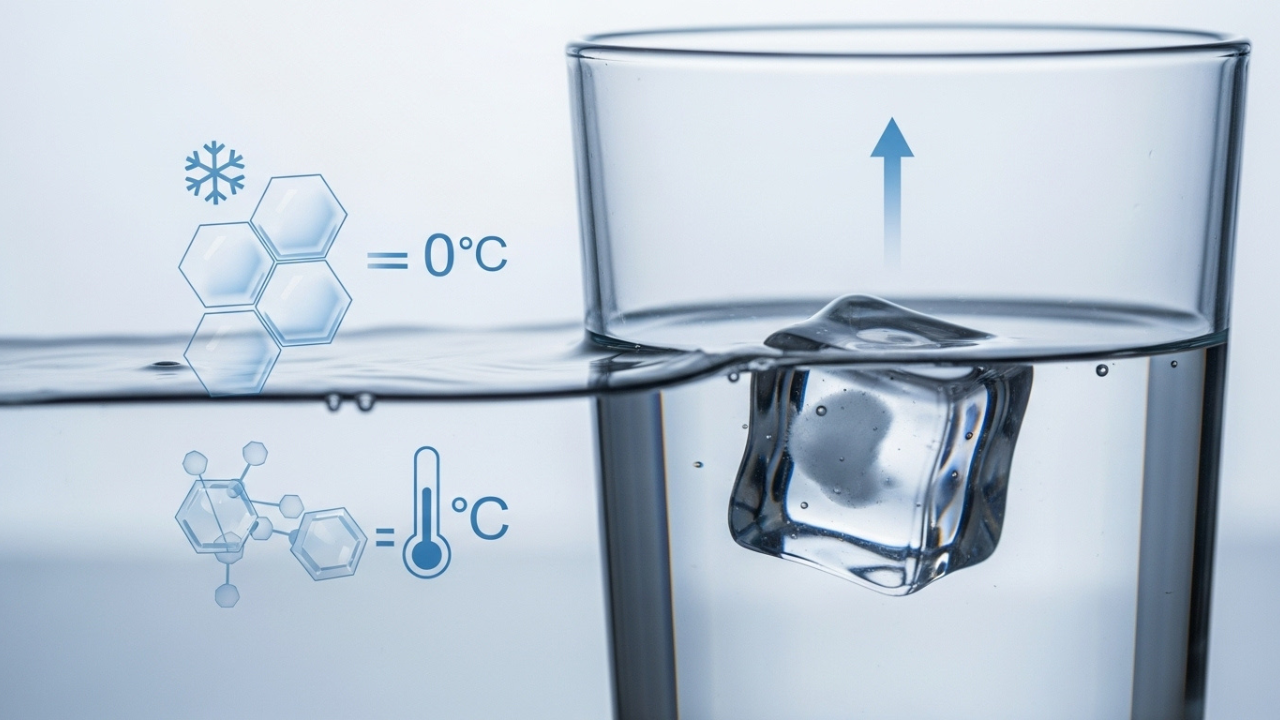
Let us talk about water now. Water, at room temperature, has a certain density which is approximately 1 gram per cubic centimeter (g/cm³). Ice is formed when water is frozen into ice and something interesting happens. The molecules in water form crystalline structure, which requires more space than liquid water, causing the ice to be less dense than water. The hydrogen bonds between water molecules force the molecules to be arranged in an open, hexagonal structure when frozen. This arrangement creates more space between the molecules which in turn makes ice less dense.
Because of the lower density of ice as compared to liquid water, ice can float. An object can float on water if it displaces a volume of water that weighs more than the object itself.
What Happens to Water as it Freezes?
While in its liquid form, water molecules are constantly interacting with each another. As water starts to cool and freeze, however, the molecules start slowing down and adopting fixed positions. The hydrogen bonds which form between water molecules, results in them being forced apart to the block-like temperature arrangements of crystal structures.
This is the reason ice in fact expands, which means that it occupies more volume than an equivalent amount of liquid water. In solid form, the molecules are arranged in a lattice structure which is not as compact as in water, hence the reduced density of ice.
This enables ice to float because it displaces a volume of water that is heavier than the ice. So, if the ice is placed in water, it will float on its surface. This is crucial in establishing the concept of ice is floating on the surface of water submerged—everything is fundamentally tied to the fact that the structure of the ice is less dense and lighter than water.
The Physics of Buoyancy: Why Ice Floats in Water
To comprehend why ice is able to float, we first need to look into a bit of detail on buoyancy, and that involves understanding Archimedes’ Principle. Archimedes’ Principle states that:
“An object immersed in a fluid (liquid or gas) experiences an upward buoyant force equal to the weight of the fluid displaced by the object.”
In simpler words, we can say, that whenever we put an object in water, it pushes back the water with the equal amount of force to the amount of water displaced—this amount of water is equal to the amount of water which gets pushed out. The object will float if its weight is lower than the upward force acting on it and sinks otherwise.
Now, if an ice cube is dropped into the water, ice cube displaces a certain volume of water. Because ice is less dense than water, it displaces enough water to counter balance its weight, which is the reason it remains at the surface of the water.
The Role of Temperature in Ice and Water’s Interaction
You have already learned that temperature is a significant factor in the kinetic state of the water and ice system. Water turns to ice at 0°C (32°F). At lower temperatures, the water molecules slow down even further and arrange themselves into the familiar hexagonal crystal structure, which makes ice less dense than liquid water.
This characteristic of water is rather exceptional; that is, its property of becoming less dense when frozen. Most materials become denser when they solidify due to the increasing intermolecular forces acting on particles. However, the structure of water molecules does modify when it freezes resulting in the formation of a less dense material. This stands as one of the exceptional instances in nature.
Why Does This Matter?
You might be wondering, “What is the fundamental significance of this?” This quality of water is incredibly crucial in nature. If ice did not float on water, lakes, rivers, and even the oceans would be very different.
For example, in the colder parts of the world, lakes and rivers would freeze from the bottom upwards, destroying the ecosystem that resides there. But because ice floats, it forms a thick protective blanket that allows life to survive beneath it even in freezing temperatures.
Ice and Water in Nature: Floating Ice in Lakes and Oceans
You might have come across an iceberg in the ocean or frozen lakes with ice on top. So, how does Iceberg not causing malfunction to marine life by sinking?
Icebergs in the Ocean
Icebergs are large blocks of ice that are found floating in water bodies; they are formed when glaciers are cut off. The reason why the Iceberg floats in water is about 90% of it is submerged which is only 10% above water, this is also how deep block is submerged. The reason floating is possible is because Ice is 9% less denser than Water meaning Ice is approximately 90% less denser than Water. Although Ice has being of a lesser density it still is required to displace a great volume of water needs to be displaced for it to float resulting in the massive Iceberg being exposed on the ocean’s surface.
Because of this reason a lot of the wasmdering water of the ocean is hidden in the surface of the water and the rest is under the surface. The under surface portion has high chances of creating dangers for ships or having an impact on the environment.
Frozen Lakes and Rivers
In regions with colder winters, Lakes and Rivers usually freeze when temperature drop below the freezing mark. The cold water does not freeze underneath the ice which maintains life in Fish and other living beings.
If the temperature sinks further it will start freezing and put every living organism in danger, but because ice floats it keeps the water below insulated and allows aquatic life to continue thriving even in the harshest winter conditions.
Can Ice Ever Sink in Water?
You may be asking: What are the conditions in which ice may sink? Yes, ice sinks, but under very particular conditions. For ice to sink, the water must be denser than the ice. This may happen under special conditions when the water’s density is high enough to overcome the buoyant force of the ice.
For instance:
Saltwater: Saltwater is denser than freshwater, and in saltwater of very high salinity, ice might sink. However, under most natural conditions (freshwater lakes or rivers), ice will float.
Very high pressure: Ice can undergo a transformation under extreme conditions such as the deep ocean. This makes the ice congruent to ‘normal’ ice, but ice in dense materials is subject to an increase in pressure. This is a very interesting application of physics but does not greatly change the everyday notions of ice floating in water when placed in a glass of water.
My Opinion| Why Ice Floats in Water and Why It’s Important
The reason ice floats in water has to do with the core concepts of density and buoyancy. Ice is less dense than liquid water on account of it’s unique molecular structure which makes it occupy more space as it freezes. That results in an upward force that enables the ice to float, hence ice is buoyant.
Knowing this concept is imperative not only for foundational physics, but for sustaining relationships between phenomena in the real world as well. From natural mechanisms that shield aquatic life during the freezing temperatures and to the carriage of icebergs through the ocean, the ability of ice to float is central to the harmony of nature.
Thus, the next time when you add an ice cube into your beverage, remember the reason why it floats. It serves as a reminder that the physical world operates in ways that we frequently overlook.





























Leave a Reply
View Comments