Explorer, envision for me a calm day in school. You are sitting at your desk with the school hot chocolate cup.
The school hot chocolate is at the right temperature that you barely need to hold it and the heat is already warming up your palms.
Now shift your focus to the metal part of the desk you’re seated on. It’s freezing even though you, along with the cup, have kept it in the same room the whole morning. Air, surroundings, does not change. What changes are the sensations.
This moment is where most students begin asking themselves – what is the scientific explanation for the difference between heat and temperature.
One makes your hand feel warm, the other is determined using a thermometer. But they seem to be related. And in science class, the teacher keeps repeating they are not the same. It would be best is we left talking about definitions for today. In turn, let’s comprehend them instead. Walk through the differences without having to rely on formulas but using physical observation.
Key Takeaways
- Heat is energy in motion—moving from warm to cool, always seeking balance.
- It gives/shows an idea of the activity of the particles within an object.
- Temperature alone does not output more heat because heat depends on the mass as well as the materials constituting it.
- The mind does not register the flow of heat and temperature, only perceives it. Thus, using the sense of touch can deceive you.
- This information allows for the formulation of constructive decisions and real-life solutions.
When You Felt Heat Without Seeing Temperature
You already have come across the concept of heat several times even before you are confident enough to stitch the letters of the word together. Example could be standing about a bonfire, without touching the flames you could sense the wind from your skin.
Another one could be picturing oneself sliding into a bathtub only to shriek because average water temperature is significantly higher than the human body’s resting temperature. In all those components, you could witness energy being exhausted, with your presence there would allow for vomitting in the funnel therefore you could claim that you come across these various situations without realizing that these states of thermal energy were present you were merely put in their influx.
Now consider the concept of temperature. You have witnessed its representation on digital displays, be it room temperature, body temperature, or even the external weather. However, temperature is not something you can feel with your hands. It is not tangible. Rather, it is a measurement. It reflects the activity taking place within the particles of a substance. While heat is concerned with motion and transfer, temperature relates to speed and equilibrium. Not so clear yet? That’s perfectly fine. We are about to help it work for you.
What Heat Really Is And How You Already Understand It
Let us begin with heat first. Heat is stransfer of thermal energy from one object to another, and in its simplest form, it is energy in motion. Heat’s direction can never change and will always move from one area to another based on the quantity in each place. Just like pouring water from a full glass into an empty one, heat makes things equal. When you hold a cup of hot chocolate, it’s not merely sitting in your hand, but flowing or streaming into it, and thus, the cup loses energy while the skin gains it. This is heat doing its job.
It’s really difficult to omit the feeling sent from heat and heat isn’t clamped to how hot something feels. A massive bowl of warm soup might have more heat than a cup of boiling water. This backward notion takes some getting used to. Take for example the pot holds more mass which means more molecules so those molecules added together make for more total energy. Heat is all about total energy content, not critical intensity, and needs to adapt at varying conditions.
Now, refocus your thoughts to the classroom. The metal surface of the desk is cold. Why? Because it is absorbing heat from your hand. Your body is warmer and the metal conducts it away quickly. It is not cold in a vacuum; it simply is a far better thief of energy. That’s always in motion. Always moving to where there is less of it and attempting to balance things out.
What Temperature Means And How It’s Different From Heat
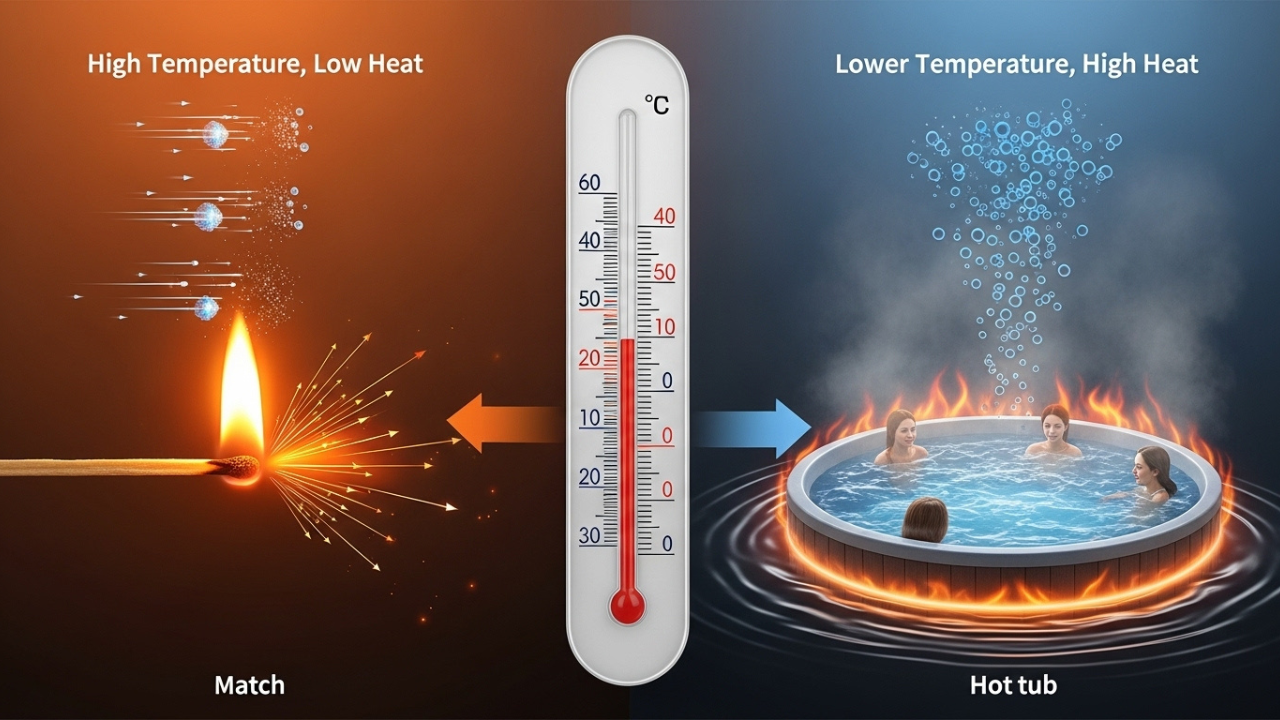
Now, we can discuss temperature. It is not a quantification of heat content, but rather a quantification of the speed of particle movement within a substance. Picture it as a speedometer and not a gauge measuring fuel. Two objects with identical temperatures may contain vastly differ amounts of energy. Therefore, a match flame and hot tub feel very different.
A match is extremely hot and the particles contained within the match do move very quickly, however, its mass is negligible, therefore the total amount of heat will be very low. The hot tub, however, is not as hot, but does possess a stoic amount of mass. Thousands of water molecules, all containing bound energy, would together would make the hot tub.
This is the point where nearly all learners stumble. They see something hot and conclude that it has more heat—and that is not correct. There is no guarantee that energy exists; it simply states that there is some energy. For example, energy stored in a small cup of boiling water is being maintained by a very small number of particles in rapid motion, while a large pool at a lower temperature may possess a larger total energy with a greater number of particles, albeit moving slower.
Type of energy that thermometers are constructed to measure is temperature. They cannot measure heat itself. Instead, they provide the pace, average kinetic energy of the particles in a substance. As a result, we can have two objects with equal temperature and yet related to the matter’s nature and quantity, they can feel different. Unlike temperature which is concrete, heat has a large variety of meanings.
A Real-Life Moment That Shows the Difference
Explorer, consider the following scenario. You are at home and put two plates of food in the microwave. One is a small slice of pizza, and the other is a full plate of rice. Both items share the same heating duration in the microwave. Upon microwave removal, the pizza blistering my mouth, while the rice remains warm to the touch. With pizza, the heat is intense and its molecules are agitated. As for rice, despite its bulk, its heat is distributed across a greater mass. While possessing higher energy, the rice does not seem as intense when consumed in one bite.
This explains the difference. Heat is the total amount of energy. In contrast, the temperature is the degree of intensity. You may have something that is boundless and does not require energy, and something that has less boundless energy which feels sharper simply because of the way it is packed and transferred. Your organs identify the transfer, not the quantity. That is why we sense and feel heat, while measuring temperature.
Why Touch Can Be Misleading
Your body can’t be a perfect system of measuring temperature. It detects the motion of energy rather than stable values. It is due to this reason why metal “feels” colder than room temperature. Metal, unlike wood or fabric, pulls energy from your skin at a much faster rate. The faster energy is transferred from you, the colder something feels. Metal does not mean it is lower in temperature; it simply means it is much better at taking heat from you.
At the same time, two objects that you wish to touch may not be similar. One may simply appear to be hotter because it is shiny or darker. However, color along with texture impact how radiant and absorbed heat is. That is why black clothing appears to feel hotter when outdoors — it absorbs radiation and in turn transfers more heat over to your body. However, it is temperature will not be higher compared to a white shirt placed in the shade. Your experience of heat stems from motion — energy reaching you rather than a measuring tool.
How Science Uses the Difference To Do Real Work
Now let us consider something more pragmatic. Engineers working with car engines manage both heat and temperature. An engine runs at a certain temperature, but its components have to either store or release a tremendous amount of heat. That’s why radiators are in place—to get rid of heat without letting the temperature get too high. The same happens with cooking.
A pan heats up very quickly but does not want to hold much heat, compared to a cast iron skillet, which warms up more gradually but stores larger amounts of energy. This is why food cooked in cast iron skillets stays hot long after turning off the stove. The food’s temperature, indeed, might match, but the heat content is greater.
This understanding is also helpful to astronauts. In space, where air is not available to transfer heat through convection, they rely solely on radiation and conduction. Spacecraft must regulate the precise amount of heat exchange—both inflow and outflow, in addition to the mentioned exterior temperature, and the energy in the environment. What they measure is temperature. What keeps them alive is heat.
My Opinion | How This Helps You Think Clearly In Science Class
Explorer, in your journey of everything physical science, this difference between heat and temperature may sound trivial, but when you grasp that heat means energy flows and temperature indicates particle motion, you simplify them on exams. More significantly, you start understanding what is actually unfolding in the world.
Now you know why water takes so long to boil yet keeps its temperature for a long period. Also, sand cools down quickly at night even after the hot day. You start understanding the unseen movement of energy; its circulation, resting places, and how better we can harness it. The most intelligent people do not simply learn definitions. They wonder about how and why. In the case of heat and temperature, the why is found in answers concerning cooking, climate, machinery, and even your own system.



























Leave a Reply
View Comments